Consistent Medical Innovation Creates Value
The key to value creation in any industry is intimately linked to the ability to consistently commercialize innovative products or services -particularly those that deliver benefits aligned with emerging trends which come to define the future of the industry. The first mover advantages that result from introducing innovative products, which solve emerging problems before they are widely recognized, allow companies to build dominant positions in rapidly growing markets.
There are few industries where innovation is more valued or urgently needed than the $220 billion global medical products market. Innovation is the life-blood of the industry. The medical supply industry has had a consistent growth rate of over 10% for the last several years. Leading companies struggle to maintain 30% of their revenues from new products introduced in the last 3 years. An innovation process that insures consistent leading edge medical solutions at low-cost will create tremendous value in medical supply markets.
Eureka Medical is a leading innovation agent that consistently and systematically finds, refines, and monetizes new product ideas developed by medical professionals. Eureka employs a unique set of innovation discovery tools to proactively attract an effective network of independent medical inventors. The company is based on a simple, but powerful proposition: A motivated inventor network, sourced from the five million independent, highly educated medical profession, can be organized into a potent innovation engine to drive medical breakthroughs and simple solutions which enhance healthcare.
Continuous Innovation Deal Flow = Remarkable Value Creation
The Opportunity
The healthcare delivery system is under tremendous pressure to identify and commercialize simple solutions quickly to lower costs, improve quality, reduce liability and eliminate preventable errors. With the convergence of scientific, materials, information, engineering and electronic technologies; innovative new breakthroughs in medical devices will play a critical role in solving the problems in healthcare and enhancing the human condition.
A special set of challenges exists with medical innovations. With over five million medical professionals practicing in hundreds of medical specialties, each with dozens of rapidly changing procedures, the matrix of opportunity is remarkably rich, broad – and complex. Change is so rapid that new technology can often make patents obsolete long before they run their course. In this environment consistent idea flow has tremendous value and can become the only reliable source of sustainable competitive advantage.
Yet the most powerful source of innovation in the space is impeded by inefficiencies. One of the biggest challenges is identifying quality ideas and getting the innovators connected to the right specialized channels with a high quality proof of concept and intellectual property protection. Another issue is matching the appropriate incentives to infuse the necessary energy and with risk mitigated and synchronized to the rewards.
Vertically organized medical products corporations often miss opportunities to capture innovation at the source – as evidenced by their record of internal innovation. For example, in analyzing the cardiology device industry over a 10-year period, Harvard Business School Professor Clayton Christensen found that that the vast majority of new devices come from independent medical professional outside the costly corporate R&D effort.
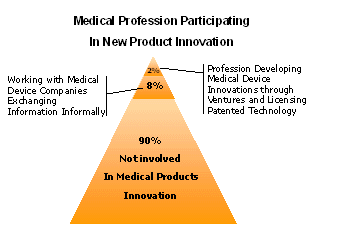
Though independent inventors-especially highly trained healthcare professionals who use medical products every day-are ideally suited to be innovators, yet they face a complex maze of challenges and staggering risks.
The complexity stems form requirements such as: FDA approval, patent concerns, regulated procedures, clinical testing, channel availability, as well as resource and focus limitations inherent in medical practice. Most medical professionals are so busy that devoting time and energy to developing and commercializing a new product is out of the question.
In addition, it’s typically very difficult for an independent inventor to gain access to innovation-buying companies or venture capital firms. Trust is also a significant barrier. With the imbalance in power, inventors are appropriately reluctant to share innovations without legal protection and negotiation guidance. The successful venture capital model targets only blockbuster product opportunities (those that have potential markets exceeding $500 million) that can be supported initially by a single product application and requires a 20% success rate to payout for investors.
A strong management team also has to be coupled with the new innovation to win venture support. This creates a risk reward balance, lack of influence on the outcome and full time focus that is uncomfortable or unacceptable for most practicing medics. Yet it is the primary avenue available for an independent medical professional to commercialize an invention. In addition, single product start-up ventures are not the ideal channel for many of the new device ideas conceived in the profession.
Just because a venture fails at commercializing a single medical device, does not necessarily mean that the medical product being commercialized was a bad idea. In many cases the new invention would likely have been commercially viable with a channel other than a single-product start-up venture.
Given these obstacles and other inefficiencies, Eureka is confident that there are at least ten value creating medical product ideas lost, for every one that is successfully commercialized. We intend to find the best of the medical solutions conceived in the profession and take them to the market, before they get lost, to create value and enhance life.
For more information on the Eureka opportunity please contact invent@eurekamed.com.
